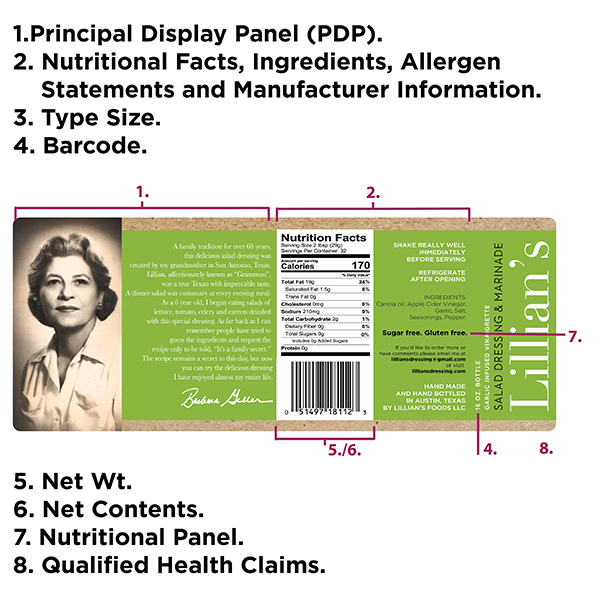
The Food and Drug Administration (FDA) had originally proposed a series of changes to the Nutrition Facts label, set to go into effect for most companies by July 26, 2018, before an update put these changes on indefinite hold. All the while, many companies have already incorporated these changes to their food labels, based on the understanding that many consumers want clearer guidelines for their food shopping. With a pending new presidential administration at the time, the career service people at the FDA took the planned changes off of hold and put them into effect as of January 1, 2021. This white paper dissects the various aspects of the FDA plans, in order to help simplify your food labeling projects.
NOTE: Manufacturers with $10 million or more in annual sales must switch to the new label by January 1, 2020; manufacturers with less than $10 million in annual food sales have until January 1, 2021, to comply.
Download the White Paper To:
- Learn what makes up the Nutrition Facts Label
- Consider inclusions of Qualified Health Claims
- Distinguish new Requirements and Exceptions
- Take next steps in your Food Labeling Program